Answer:
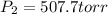
Step-by-step explanation:
Hello there!
In this case, when we have a gas that is undergoing a change in both pressure and temperature, we utilize the Gay-Lussac's equation as shown below:
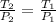
Thus, since we are given the initial pressure and temperature and the final temperature, we can compute the final pressure as shown below:
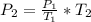
So we plug in, by making sure the temperatures are in kelvins, to obtain:
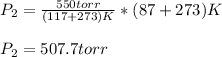
Best regards!