Answer
The mass/volume percent = 16/50 x 100% = 32%
Step-by-step explanation
Given:
Mass of NaCl, m = 16.0 g
Volume of solution, v = 50.0 mL
What to find:
The mass/volume percent.
Solution
The mass/volume percent of the solution can be calculated using the formula below
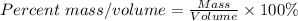
The mass/volume percent = 16/50 x 100% = 32%