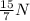Answer:
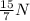
Step-by-step explanation:
We already know that the mass number of an atom is the sum of the number of protons and the number of neutrons.
So, the mass number of this isotope is;
Number of protons = 7
Number of neutrons = 8
Mass number = 7 + 8 = 15
Hence, the isotope is;