Answer:
Step-by-step explanation:
Hi there!
We have,
1 mole of Al = 6.23*
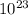 atoms
atoms
Also given no. of atoms = 3.90 x
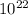 atoms
atoms
Now,
6.23*
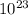 atoms= 1 mole
atoms= 1 mole
1 atom =
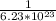 moles
moles
or, 3.90 x
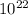 atoms =
atoms =
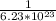 *3.90 x
*3.90 x
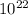 moles
moles
Therefore, 3.90 x
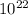 atoms has 0.0626 mols of Al.
atoms has 0.0626 mols of Al.
Hope it helps!