Answer:
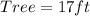
Explanation:
Given
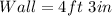
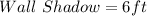
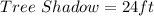
Required:
Determine the height of the tree
To do this, we make use of the following equivalent ratios
Wall : Wall Shadow = Tree : Tree shadow
Substitute values for Wall, Wall Shadow and Tree Shadow
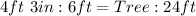
Convert all units to ft
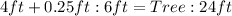
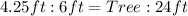
Convert to fractions
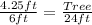
Cross Multiply
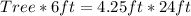
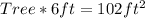
Make Tree the subject
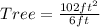
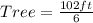
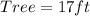
Hence, the tree is 17ft tall