Answer:
0.591 g of magnesium phosphate is the theoretical yield.
Magnesium nitrate is the limiting reactant.
Step-by-step explanation:
Hello!
In this case, since the balanced reaction turns out:
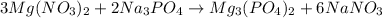
Next, we compute the grams of magnesium phosphate yielded by each reactant, considering the present mole ratios and molar masses:
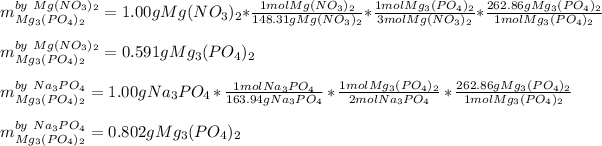
Thus, we infer that the correct theoretical yielded mass is 0.591 g as magnesium nitrate is the limiting reactant for which it produces the fewest grams of product.
However, is not possible to compute the percent yield since no actual yield is given, and must be provided or indicated by the problem or an experiment and it not here, nevertheless, you may compute the percent yield by dividing the actual yield by the theoretical and then multiplying by 100:
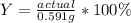
Best regards!