Answer:
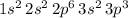 .
.
Step-by-step explanation:
In an atom of a given element, the number of electrons should be the same as the atomic number of that element.
In this question, the atom with atomic number
 will have
will have
 electrons.
electrons.
In electron configurations, the number and the letter (e.g.,
 ) denote atomic orbitals. The superscript (e.g., the "
) denote atomic orbitals. The superscript (e.g., the "
 " in
" in
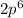 ) denote the number of electrons in those orbitals.
) denote the number of electrons in those orbitals.
The
 orbital of each shell (
orbital of each shell (
 ,
,
 , etc.) can hold up to
, etc.) can hold up to
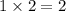 electrons. The
electrons. The
 orbitals of each shell (e.g.,
orbitals of each shell (e.g.,
 ,
,
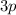 ) can hold up to
) can hold up to
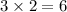 electrons.
electrons.
The electrons will fill atomic orbitals with the least amount of energy before going to the next (the Aufbau Principle.) The first few orbitals are filled in the order of
 ,
,
 ,
,
 ,
,
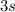 ,
,
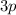 ,
,
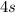 ,
,
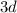 .
.
For the atom in this question (from orbitals of lower energy to higher energy):
 orbital is completely filled
orbital is completely filled
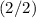 .
.
(There is no
 orbital in the first shell)
orbital in the first shell)
 orbitals are completely filled
orbitals are completely filled
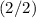 .
.
 orbitals are completely filled
orbitals are completely filled
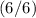 .
.
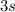 orbitals are completely filled
orbitals are completely filled
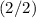 .
.
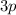 orbitals are only partially filled
orbitals are only partially filled
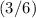 since there are exactly
since there are exactly
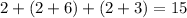 electrons in this atom.
electrons in this atom.
Therefore, the electron configuration of this atom would be
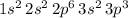 .
.