Explanations:
Exothermic reactions are reactions that release heat to the surroundings during the chemical reactions. The enthalpy change for an exothermic reaction is negative because the the energy the product is less than that of the reactant.
For exothermic reactions:
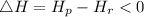
The enthalpy profile shows how the energy changes during a reaction. The enthalpy profile for an exothermic reaction is as shown below:
From the diagram, the definition of the symbols are
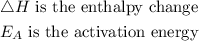
The presence of any catalyst in a chemical reaction speeds up the rate of the chemical reaction by lowering the activation energy in the reaction profile.