Let's see first the molar mass of NO2, using the periodic table. You can see that 16 g/mol is the molar mass of oxygen and 14 g/mol is the molar mass of nitrogen. In the formula, there are 2 atoms of oxygen and one atom of nitrogen, so we need to do the algebraic sum to find this molar mass, like this:
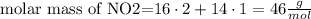
But in the problem, they're telling us that the molar mass is 92 g/mol, so we need to multiply each atom by two, because 46 x 2 = 92 g/mol, so the compound would be N2O4. Let's see its molar mass:
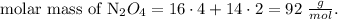
The answer is B, N2O4.