The final temperature : T₂ = 680.625 K
Further explanation
Given
V₁=240 ml
T₁ = 90 + 273 = 363 K
V₂ = 450 ml
Required
The final temperature
Solution
Charles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature
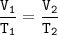
Input the value :
T₂ = V₂T₁/V₁
T₂ = 450 x 363 / 240
T₂ = 680.625 K