Answer:
570. g Fe₂(SO₃)₃
General Formulas and Concepts:
Atomic Structure
- Reading a Periodic Table
- Compounds
Stoichiometry
- Using Dimensional Analysis
- Molar Ratio
Step-by-step explanation:
Step 1: Define
1.62 moles Fe₂(SO₃)₃
Step 2: Identify
Molar Mass of Fe: 55.85 g/mol
Molar Mass of S: 32.07 g/mol
Molar Mass of O: 16.00 g/mol
Molar Mass of Fe₂(SO₃)₃: 2(55.85) + 3(32.07) + 9(16.00) = 351.91 g/mol
Step 3: Convert
Use dimensional analysis.
- Set up:
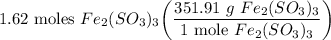
- Evaluate [Cancel out units]: 570.094 g Fe₂(SO₃)₃
- [Round] Significant Figures: 570.094 g Fe₂(SO₃)₃ ≈ 570. g Fe₂(SO₃)₃
Topic: Chemistry
Unit: Atomic Structure/Stoichiometry