Final answer:
The most sensible answer to the Herbert analogy is 'a) How strong is the storm?' as it refers to the concentration of H+ ions that determines the pH level. The pH is a negative logarithm of the hydrogen ion concentration, with a pH of 7.0 considering neutral for pure water.
Step-by-step explanation:
The pH scale is a logarithmic measure used to represent the acidity of a solution, which is determined by the concentration of hydronium (H3O+) ions. To answer the analogy question regarding Herberts and a storm on a lake, the most sensible response would be a) How strong is the storm? This is because the 'strength of the storm' would correlate to the concentration of H+ ions in the solution, thus determining the pH level, akin to knowing how many Herberts are drowning. The pH scale compresses this wide range of hydrogen ion concentrations into manageable numbers. A low pH indicates a high concentration of hydrogen ions, denoting an acidic solution, while a high pH indicates a low concentration, denoting a basic solution.
A neutral pH value is 7.0, which is the case for pure water. The pH is calculated as the negative of the base 10 logarithm (negative logarithm) of the hydrogen ion concentration, such as with pure water, which has a concentration of 1 ×
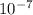 moles H+ ions per liter. Therefore, the pH of water is calculated as -log10(1 ×
moles H+ ions per liter. Therefore, the pH of water is calculated as -log10(1 ×
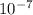 ), which equals 7.0.
), which equals 7.0.