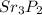Answer: The components are Strontium (Sr) and Phosphorous (P). The symbol for the element that forms the positive cation is
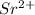 . The symbol for the element that forms the negative anion is
. The symbol for the element that forms the negative anion is
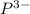 . The subscript on the cation in the neutral formula is 3 and the subscript on the anion in the neutral formula is 2.
. The subscript on the cation in the neutral formula is 3 and the subscript on the anion in the neutral formula is 2.
Step-by-step explanation:
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non metals.
Here in strontium phosphide , element strontium is having an oxidation state of +2 called as
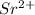 cation and element phosphorous
cation and element phosphorous
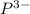 is an anion with oxidation state of -3. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral strontium phosphide
is an anion with oxidation state of -3. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral strontium phosphide