Answer:
the average kinetic energy of the 10 molecules is 10 J.
Step-by-step explanation:
Given;
energy on one molecule in motion, E = 100 J
number of molecules, n = 10
(A) The average kinetic energy of the 10 molecules
since the remaining 9 molecules are at rest, their kinetic energy = 0
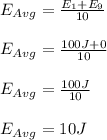
Therefore, the average kinetic energy of the 10 molecules is 10 J.