Step 1
A dilution is calculated as:
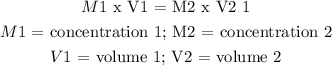
----------------------
Step 2
Information provided of lithium acetate:
M1 = 2.6 M
V1 = 350 mL
-----
M2 = unknown
V2 = 800 mL
-----------------------
Step 3
Procedure: from (1)
M1 x V1/V2 = M2
2.6 M x 350 mL/800 mL = M2
1.1 M approx. = M2
Answer: M2 = 1.1 M