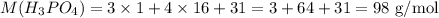Step 1 - Understanding the reaction and finding its stoichiometry
The reaction between Magnesium oxide and Phosporic acid is as follows:
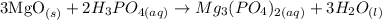
We can see that 3 moles of MgO react with 2 moles of H3PO4. We can convert this relation in moles to a relation in mass by multiplying each number of moles by the respective molar mass of the substance (40 g/mol for MgO; 98 g/mol for H3PO4):
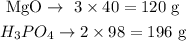
We know, therefore, that 120 g of MgO react with 196 g of H3PO4. This is like a cake recipe: it is a fixed proportion. It is like you knew how many eggs and sugar you would need so they can make a cake and nothing is left.
Step 2 - Using the stoichiometry of the reaction to solve the exercise
Now that we already know the recipe, we can use it to predict how much we will to make more or less cake. Remember: the proportion between the reactants is always a fixed one.
Since we want to know how many grams of H3PO4 are necessary to completely react with 2357 g of MgO, we can set the following proportion:
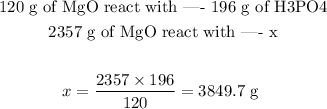
We would need, therefore, 3849.7 g of MgO to completely react with the given mass of H3PO4.
note: To calculate the molar mass of a substance, we just have to sum up the molar masses of the atoms, multiplying each molar mass by the number of times each atom appears in the formula. The molar mass of the atoms can be found in the periodic table.
Let's use H3PO4 as an example. Looking at a periodic table, we find the molar masses:
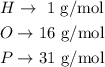
The molar mass of H3PO4 would be thus: