Answer:
True
Step-by-step explanation:
The electron configuration of calcium (Ca) is 1
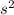 2
2
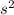 2
2
 3
3
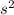 3
3
 4
4
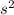 . This tells us that there are 2 electrons in the 4th energy shell of an atom with 8 otyher electrons distributed as described in the electron configuration among the energy shells and orbitals (s, p, d, and f).
. This tells us that there are 2 electrons in the 4th energy shell of an atom with 8 otyher electrons distributed as described in the electron configuration among the energy shells and orbitals (s, p, d, and f).