Answer:
0.0000076 grams
Step-by-step explanation:
We're given the half life of Tritium to be 12.3 years. In order to find out the amount of substabce remaining:
Let's first find how many 'half lives' are in 250 years.
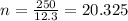
Now what is half life? It means the time taken for a given quantity of an element to lose half it's mass.
So in 12.3 years we can find that The amount of 250 g of Tritium will be 250/2 = 125 g. In 24.6 years we'll have 125/2 = 62.5 g
So now we can devise a formula:
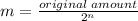
Where m is the remaining amount and n is th number of half lives in the time given.
Using this formula we can calculate:
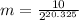
Doing this calculation we get:
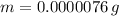
As we can see a very small value remains.