4.58*10^-3 moles of Al2O3 will be produced.
1st) It is necessary to write the balanced equation:
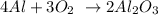
From the balanced equation we know that 4 moles of Al react with 3 moles of O2.
2nd) Now it is necessary to find out the limiting reactant of the reaction, using the 6.38*10^-3 moles of O2 and 9.15*10^-3 moles of Al given.
With the moles in the balanced equation and the moles given we can calculate the limiting reactant:
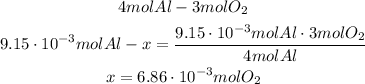
Now we can see that 9.15*10^-3 moles of Al will need 6.86*10^-3 moles of O2, but we only have 6.38*10^-3 moles of O2, so as the amount of oxygen is not enough, O2 will be the limiting reactant.
3rd) Using the excess reactant (Al), we can calculate the moles of Al2O3 that will be produced:
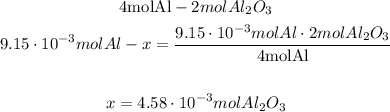
Finally, 4.58*10^-3 moles of Al2O3 will be produced.