Answer:
0.225 moles
Step-by-step explanation:
Looking at the balanced equation, 4 moles of Fe(s) react with 3 moles of
 (g) to produce 2 moles of
(g) to produce 2 moles of
 (s). In other words, for every 3 moles of
(s). In other words, for every 3 moles of
 (g) used, there are 2 moles of product
(g) used, there are 2 moles of product
 (s). The mole ratio is therefore 3:2. In other words, you the moles of
(s). The mole ratio is therefore 3:2. In other words, you the moles of
 by
by
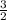 to find the moles of product.
to find the moles of product.
There are 0.15 moles of
 , so the moles of product should be 3/2 of this according to the molar ratio.
, so the moles of product should be 3/2 of this according to the molar ratio.
0.15 *
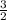 = 0.225
= 0.225
Your answer is 0.225 moles.
Hope this helps!