We have the following balanced equation:
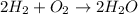
They give us the moles of water and ask us to find the moles of O2 that must be consumed.
To calculate the moles of O2 we must calculate the ratio O2 to H2O, we do this by identifying the coefficients of each molecule. For O2 we have coefficient 1 and for water coefficient 2. Therefore, the ratio O2 to H2O will be 1 /2
So, the moles of O2 will be:
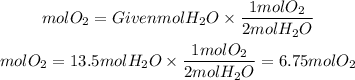
In the reaction were consumed 6.75 moles of O2