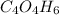To calulate the empirical formula we have to divide the mass of each component by its atomic mass, let's write down the atomic mass of each component:
Mm of O = 16 g/mol; Mm of C = 12 g/mol and Mm of H = 1 g/mol
Now we divide the measured mass of each component by its atomica mass so we can obtain a relative number of moles:
Oxygen:
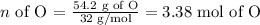
Carbon:
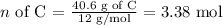
Hydrogen
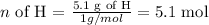
Now we divide each value by the lower number obtained in this calculation (this calculation is called normalization) in this case both C and O are both the lower number 3.38 mol:
Oxigen:
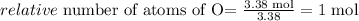
Carbon:
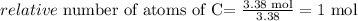
Hydrogen:
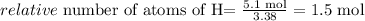
These values are the values of the empirical formula:
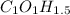
To avoid the use of decimal points in the formula it can also be expresed like:
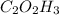
Part 2
The empirical equation gives the proportion of the atoms pressnt in the molecule but do not give the actual number of atoms of each component. To calculate the actual number of atoms we calculate the molar mass of the empirical equation by adding mass all the atoms:
Mm empirical = 2X12 + 2X16+3x1 = 59 g/mol
Now we divide the real molar mas by the empirical molar mass:
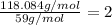
this is the number of times that the empirical molecule is repited in the real molecule so the real molecule is: