General Stoichiometry
We must understand the relationship between moles, molar mass and mass to be able to solve questions with them:

 = moles
= moles
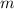 = mass
= mass
 = molar mass
= molar mass
Solving the Question
We're given:
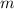 = 35.2 g
= 35.2 g
 = sum of Mg, S and O4: 120.368 g/mol (taken from Chemspider)
= sum of Mg, S and O4: 120.368 g/mol (taken from Chemspider)
Apply the formula:


Answer
0.292 mol