We have the balanced equation of the reaction:
NaOH (aq) + HCl (aq) → NaCl (aq) + H2O (l)
Now, to calculate the moles of water needed, we must look at the stoichiometric coefficients of the reaction. The stoichiometric coefficients are the numbers that come before the molecule.
In this case, all the molecules have a stoichiometric coefficient equal to 1, that is, the ratio between all the compounds is 1. Therefore, the moles of water needed will be:
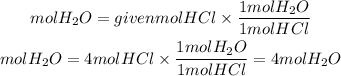
Answer: When there are 4 moles of HCl, it will be produced 4 moles of H2O