ANSWER
The wavelength of the photon is 0.00669 nm
Explanation:
Given information
The energy of the photon = 2.97 x 10^-19J
Let x represents the wavelength of the photon
The first thing to do is to establish the relationship between energy and photon
Recall that,
E = hf --------------- equation 1
Where
h = Planck's constant
f = frequency
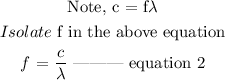
The next step is to substitute the value of f into equation 1, then, we have the below equation

Recall that,
h = 6.626 x 10^-34 J
c = 3 x 10^8 m/s
To find the wavelength, we need to substitute the above data into equation 3
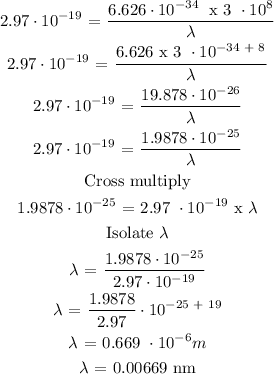
Therefore, the wavelength of the photon is 0.00669 nm