Answer:
one mole of oxygen will produce
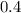 moles of water
moles of water
Step-by-step explanation:
The balanced chemical reaction is as follows -
2 C2H2 + 5 O2 --> 4 CO2 + 2 H2O
As we can see in this reaction
5 mole of oxygen (on the reactant side) will produce 2 mole of water (on the product side)
Hence, one mole of oxygen will produce
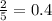 moles of water
moles of water