Answer:
0.2mol/dm³
Step-by-step explanation:
Given parameters:
Mass of Na₂CO₃ = 10.6g
Volume of solution = 500mL
Unknown:
Molarity of solution = ?
Solution:
The molarity of a solution can be determined using the expression below:
Molarity =
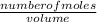
Number of moles =
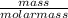
Molar mass of Na₂CO₃ = 2(23) + 12 + 3(16) = 106g/mol
Number of moles =
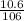 = 0.1mol
= 0.1mol
Convert mL to dm³;
500mL will give 500 x 10⁻³dm³or 0.5dm³
So;
Molarity =
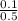 = 0.2mol/dm³
= 0.2mol/dm³