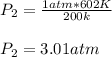Answer: The new pressure is 3.01 atm
Step-by-step explanation:
Lets used the ideal gas formula: PV = nRT
P = pressure, V = volume, n = moles, R = universal gas constant, T = temperature (in kelvin)
In this problem we are given that the change in temperature and we need to find the new pressure, when the initial pressure is 1 atm. All other variables are constant.
We can rewrite the ideal gas formula to suite this problem by saying that
P1/T1 = P2/T2
Now lets set up the equation to solve for P2
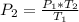
Now lets plug in the values.
P1 represents the initial pressure: 1 atm
T1 represent the inital temperature: 200k
T2 represents the final temperature: 602k
We are solving for the P2, the final (new) temperature.