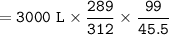
Further explanation
Given
3000 L of gas at 39°C and 99 kPa to 45.5 kPa and 16°C,
Required
the new volume
Solution
Combined with Boyle's law and Gay Lussac's law
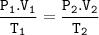
T₁ = 39 + 273 = 312
T₂ = 16 + 273 = 289
Input the value :
V₂ = (P₁V₁.T₂)/(P₂.T₁)
V₂ = (99 x 3000 x 289)/(45.5 x 312)
or we can write it as:
V₂ = 3000 L x (289/312) x (99/45.5)