Heat required = = 126.42 kJ
Further explanation
Given
1.96 mol NaHCO₃(s)
Required
Heat needed
Solution
Reaction( decomposition of Baking soda (NaHCO₃) :
2NaHCO₃ (s) + 129 kJ ⇒ Na₂CO₃ (s) + H₂O (g) + CO₂ (g)
For 2 mol NaHCO₃ , heat needed = 129 kJ
conversion factor :
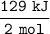
So for 1.96 mol :
= 1.96 mol x (129 kJ/2 mol)
= 126.42 kJ