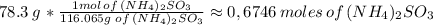Answer:
0,6746 moles of ammonium sulfite
Step-by-step explanation:
(NH₄)₂SO₃ - ammonium sulfite

Atomic weight of each element:
N = 14.00 * 2 = 28
H = 1.00 * 8 = 8
S = 32.065 * 1 = 32.065
O = 16 * 3 = 48
Total atomic weight = 28 + 8 + 32.065 + 48
Total atomic weight = 116,065 gr/mol