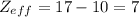Answer: A.
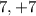
Step-by-step explanation:
Valence electrons are the number of electrons constituting the outermost shell of an atom. It is also defined as the electrons that take part in the formation of a chemical bond.
The atomic number of chlorine is 17 as it has 17 protons and the electronic configuration will be 2,8,7. Thus the electrons present in the outermost shell are 7 and hence the valence electrons are 7.
Effective nuclear charge is the net positive charge experienced by valence electrons. It is calculated by: Zeff = Z – S
where Z = atomic number , S = number of shielding electrons.
Thus for Chlorine ,