Answer:
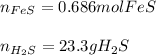
Step-by-step explanation:
Hello!
In this case, according to the given chemical reaction, it is possible to apply the following stoichiometric setups in order to compute the moles of reacting FeS and the grams of produced H2S, given the mass of HCl (molar mass = 36.46 g/mol) as shown below:
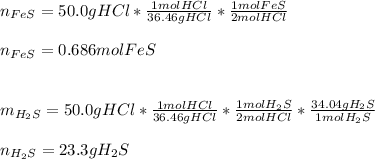
Best regards!