Answer:
No. of moles = 0.0685
Step-by-step explanation:
Given mass, m = 15.5g
We need to find the number of moles in 15.5 g of CaSO₄.5H2O
First, we find the mass of CaSO₄.5H2O.
M = (1×40)+(1×32)+(4×16)+(5×18)
M = 226 g/mol
We know that,
Number of moles = given mass/molar mass
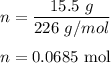
Hence, there are 0.0685 moles in 15.5 g of CaSO₄.5H2O.