Step 1 - Understanding what is ionization energy
Ionization energy is, by definition, the amount of energy required to remove an electron from an atom is its gaseous state.
Therefore, the closest the electron is to the nucleus, the hardest it is to remove it due to electrostatic-like interactions. We could expect thus that bigger atoms will have lesser ionization energies.
As a consequence, the ionization energy decreases along the family, but increases along the period due to radio decreasing.
Step 2 - Obtaining the electronic configuration for N and Li
The eletronic configuration for each element is:
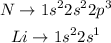
We can see that, by removing one electron from each atom, we get the following electronic configurations:
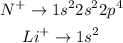
Note that Li attains a full-shell configuration, becoming extremely stable. Therefore, we expect its ionization energy to be lower than N.
In short, N has a higher ionization energy.