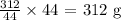Answer:
Step-by-step explanation:
Here, we want to get the mass of carbon (iv) oxide produced
We start by writing the equation of reaction as follows:
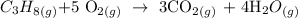
Now, we need to get the mass of propane that reacted
We can get that by multiplying the density of propane by its given volume
Mathematically, we have that as:
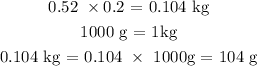
From here, we get the actual number of moles of propane that reacted
We can get that by dividing the mass by the molar mass of propane
The molar mass of propane is 44 g/mol
The number of moles is thus:
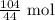
From the balanced equation:
1 mole of propane gave 3 moles of carbon (iv) oxide
104/44 mol will give x moles
We have the value of x as:
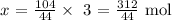
To get the mass of carbon (iv) oxide produced, we multiply the number of moles above by the molar mass of carbon (iv) oxide
The molar mass of carbon (iv) oxide is 44 g/mol
Thus, we have the mass as: