Answer:
27.95L
Explanations:
According to Charle's law, the volume of a given mass of a gas is directly proportional to absolute temperature provided that the pressure is constant. Mathematically;
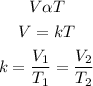
where
V1 and V2 are the initial and final volume respectively
T1 and T2 are the initial and final temperature respectively (in Kelvin)
Given the following parameters
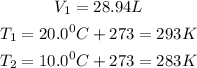
Required
Final volume V₂
Substitute the given parameters into the formula to have:
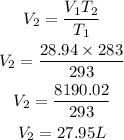
Hence the new volume of the neon gas is 27.95L