1) List the known and unknown quantities.
Total pressure: 73.2 atm.
Volume: 24.0 L.
Temperature: 264.7 K.
Partial pressures
Sample: Br2
Pressure: 9.0 atm.
Sample: Cl2.
Pressure: 682.5 InHg
Sample: H2.
Pressure: unknown.
2) Partial pressure of H2.
2.1- List the known and unknown quantities.
Partial pressures
Sample: Br2
Pressure: 9.0 atm.
Sample: Cl2.
Pressure: 682.5 InHg
Sample: H2.
Pressure: unknown.
2.2- Set the equation.
Dalton's law. This law states that the total pressure of a gas is equal to the sum of the individual partial pressures.
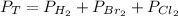
2.2.1. Convert InHg to atm.
29.9 InHg = 1 atm
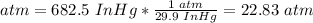
2.3- Plug in the known values and solve for P(H2).
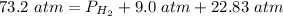
.
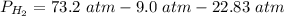
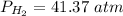
3) Moles of H2
3.1- List the known and unknown quantities.
Pressure: 41.37 atm.
Volume: 24.0 L.
Temperature: 264.7 K.
Pressure: 41.37 atm
Ideal gas constant: 0.082057 L * atm * K^(-1) * mol^(-1)
3.2- Set the equation.

3.3- Plug in the known quantities and solve for n (moles).
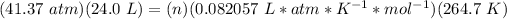
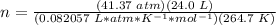
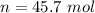
There are 45.7 moles of H2.
.