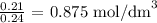Answer:
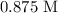
Explanation;
Here, we want to calculate the molarity of the solution
To calculate the molarity, we use the following formula:
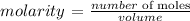
To calculate the number of moles, we have to divide the mass by the molar mass
The molar mass of sodium hydroxide is 40 g/mol
Thus, we have the number of moles as:
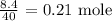
The volume to use would be in L
To get this, we divide the given volume by 1000
Thus, we have the volume in liters as:
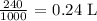
Finally, we have the molarity as: