Answer:
The total energy reaction would be -698 kJ/mol.
Step-by-step explanation:
The formula to calculate the enthalpy change of a reaction is the following:
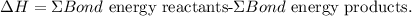
Let's calculate the bond energy for each reactant:
- CH4: As we have 4 bonds of H - C, the bond energy is 4 x 412 kJ/mol = 1648 kJ/mol.
- O2: As we have 1 bond of O = O, but we have 2 moles of O2, we just multiply the bond energy of O = O by two: 2 x 496 kJ/mol = 992 kJ/mol.
Now, let's calculate the bond energy for each product:
- CO2: As we have 2 bonds of C - O (O - C - O), the bond energy would be 2 x 743 kJ/mol = 1486 kJ/mol.
- H2O: As we have 2 bonds of O - H (O - H - O), and 2 moles of H2O, we multiply the bond energy by the number of bonds, and the number of moles: 2 x 2 x 463 kJ/mol = 1852 kJ/mol.
Finally, we just apply the given formula, like this:
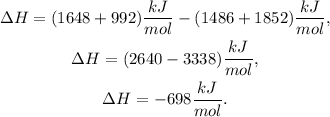
The total energy reaction would be -698 kJ/mol.