Step-by-step explanation:
The given reaction equation is as follows.
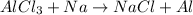
To balance this equation, we multiply Na by 3 on reactant side and NaCl by 3 also.
Thus, the balanced equation is as follows.

The oxidation and reduction reaction will be as follows.
Oxidation:
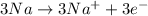
Reduction:
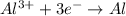
Thus, we can conclude that oxidation state of sodium changes from 0 to +1.