Answer : The correct option is, pressure.
Explanation :
The ideal gas equation is,
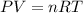
where,
P = pressure of the gas
V = volume of the gas
n = number of moles of gas
T = temperature of the gas
R = gas constant
The value of 'R' has several different values which are :





That means, the value of 'R' is different due the change in the pressure value and all the variables (temperature, volume and moles) are constant.
Hence, the correct option is, pressure.