The first step to solve this question is to state the combustion reaction for nonane:
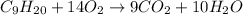
After this, we have to convert the given masses of nonane and oxygen to moles using their molecular masses:
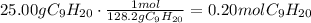
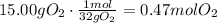
Use the stoichiometric ratio to determine the number of moles of oxygen that react with 0.20 moles of nonane.
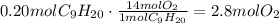
From this, we can conclude that the limiting reactant of the reaction is oxygen, which means that we have to base our calculations on the amount of oxygen that reacts. Use the stoichiometric ratio of oxygen to water to find the amount of water produced:
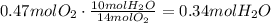
Convert the amount of moles to grams using its molecular mass:
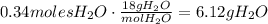
To find the percent yield, divide the actual amount of water produced by the theoretical amount of water produced and multiply by 100:
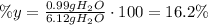
The percent yield is 16.2%.