Answer: In the following equation,
 is considered as a binary compound.
is considered as a binary compound.
Explanation: A binary compound is considered as a compound having 2 elements only. In the following chemical equation:
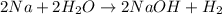
There are 2 compounds present because compound is defined as the chemical combination of two or more elements in a fixed ratio.
In the equation, compounds are:
 and
and

 consists of 2 elements which are hydrogen and oxygen and hence is considered as a binary compound.
consists of 2 elements which are hydrogen and oxygen and hence is considered as a binary compound.
 consists of 3 elements which are sodium, hydrogen and oxygen and hence, cannot be considered as a binary compound.
consists of 3 elements which are sodium, hydrogen and oxygen and hence, cannot be considered as a binary compound.