Answer: The two different neutral isotopes of same element will have Atomic Number in common.
Explanation:
Isotopes are defined as the species which have same atomic number but different atomic mass. For example:
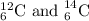 are the isotopes of same element carbon having different atomic mass.
are the isotopes of same element carbon having different atomic mass.
General form to write an isotope:

where, A = Atomic mass of the element
Z = Atomic number of the element
X = Element
As, the value of A is different for two isotopes and Z is same, so two different neutral isotopes of the same element have Atomic Number in common.