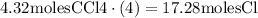So, the first thing we need to do, is to take a look at the compound's formula.
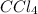
This formula tells us that:
The 4 behind the chlorine means that there are 4 chlorine atoms per molecule. We could also say that there are 4 times as many chlorine atoms as there are carbon tetrachloride (CCl4) molecules.
So, if we have 4.32 moles of CCl4, then we will have 4 times as many chlorine atoms: