The first step to solve this question is to balance the given equation:
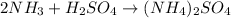
The next step is to convert the mass of H2SO4 to moles, using the molar mass of H2SO4 (98g/mol).
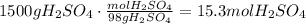
Now, use the stoichiometric ratio given by the reaction to define how many moles of NH3 react with 15.3 moles of H2SO4:
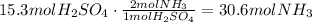
Finally, use the ideal gas law to determine the volume of the gas at 24°C (297.15K) and 25atm:

The volume of NH3 needed is 29.82L.