Step 1 - Reading a chemical equation
In order to properly 'read' a chemical equation, we must know that the bigger numbers, those that come before the formulas of each susbtance, represent a quantity in moles.
The given chemical equation could be read thus as:
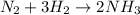
one mole of N2 react with 3 moles of H2 producing 2 moles of NH3
Step 2 - Finding how many moles of N2 are needed
Reading a chemical equation is like obtaining its recipe: we discover the proportion between the substances, and we can use it to predict how much we wll need if we 'change' the recipe.
Since we want to produce 5 moles of NH3, we can use this proportion:
one mole of N2 produces 2 moles of NH3
Therefore:
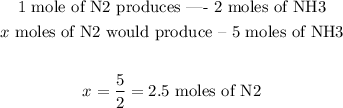
We would need thus 2.5 moles of N2.