When we want to calculate the number of molecules, we need to take into consideration the relation between mole and number of molecules/atoms. One mole is, by definition, 6*10^23 entities, be they atoms or molecules.
In this case, 1 mole of SiO2 corresponds to 6*10^23 molecules of SiO2. By using this relationship, we can discover how many moles there are in 3.4*10^25 molecules. Let's set the following proportion:
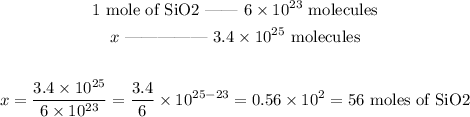
So, 3.40*10^25 molecules of SiO2 correspond to 56 moles of SiO2.