Answer: 56.9g of oxygen would be produced when 109g of sodium chlorate undergo a breakdown reaction
Step-by-step explanation:
The question requires us to calculate the mass of oxygen (O2) when 109g of sodium chlorate (NaClO4) is broken down into oxygen and sodium chloride (NaCl).
The balanced chemical equation for the breakdown reaction of NaClO4 can be written as:
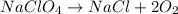
Considering this chemical equation, we can see that 1 mol of NaClO4 is necessary to produce 2 moles of O2. Therefore, we can calculate how much O2 would be produced when 109g of NaClO4 are used.
First, we need to determine the number of moles of NaClO4 that corresponds to 109g of this compound. For that, we'll need to calculate the molar mass of NaClO4 (the atomic masses of Na, Cl and O are 22.99, 35.45 and 15.99 amu, respectively):
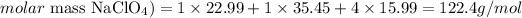
And we can calculate the number of moles of 109g of NaClO4 as:
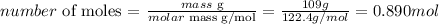
Therefore, 0.890 moles of NaClO4 were used in the reaction.
Using the stoichiometry of the reaction (1 mol of NaClO4 produces 2 moles of O2), we can calculate the number of moles of O2 that would be obtained from 0.890 moles of NaClO4:
1 mol NaClO4 ------------------------ 2 mol O2
0.890 mol NaClO4 ---------------- x
Solving for x, we'll have:
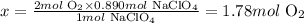
Therefore, 1.78 moles of O2 would be produced when 109g of NaClO4 react.
We can convert the calculated number of moles of O2 to its correspondent molar mass as it follows, knowing that the molar mass of O2 is 31.98g/mol:
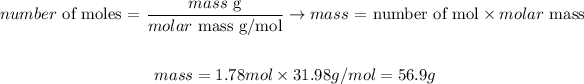
Therefore, 56.9g of oxygen would be produced when 109g of sodium chlorate undergo a breakdown reaction.